News
20/12/2023
Gantenerumab in Early Alzheimer’s: Pursuing Amyloid Reduction and Clinical Impact
- anti Aβ-antibody gantenerumab tested in phase 3 clinical trial
- antibody successfully removed Aβ plaques
- gantenerumab did not slow cognitive decline in participants
In the ongoing search for a treatment for Alzheimer’s disease, recent developments have unveiled a group of promising drugs that are based on the ‘amyloid-cascade-hypothesis’ introduced 30 years ago. Recently, the application of the monoclonal antibodies lecanemab and donanemab, designed to target amyloid-beta (Aβ), have demonstrated an effective amyloid removal and a moderate potenial to slow down clinical decline [1,2].
The present study focuses on yet another anti-Aβ monoclonal antibody called gantenerumab, which underwent two phase 3 trials (Graduate I and II) targeting Aβ in early Alzheimer’s disease. Enrolling 1,965 participants (aged 50 to 90) showing mild cognitive AD-related symptoms, the trials explored cognitive development and a possible reduction of amyloid burden after gantenerumab treatment.
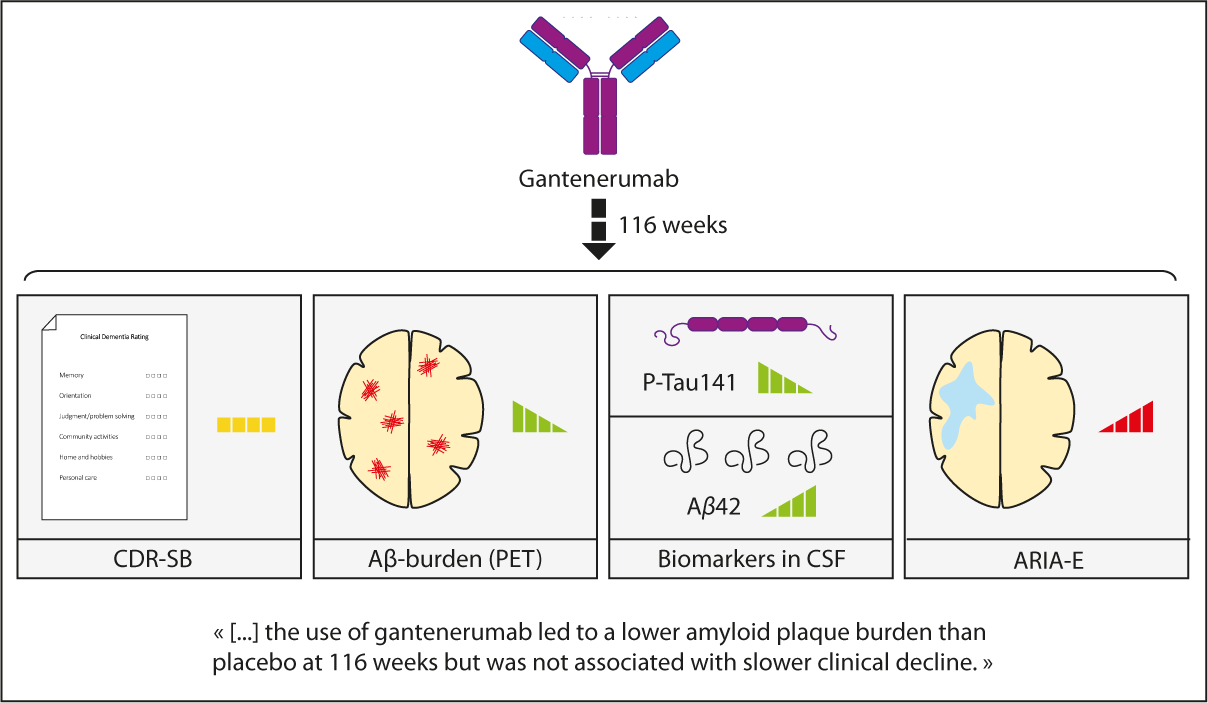
While the CDR-SB score change at week 116 with gantenerumab suggested some trend towards slower decline compared to placebo, the difference was not statistically significant (GRADUATE I: -0.31, P = 0.10; GRADUATE II: -0.19, P = 0.30).
Gantenerumab did demonstrate a notable reduction in amyloid plaque burden on PET, achieving amyloid-negative status in GRADUATE I and II. Cerebrospinal fluid (CSF) analysis also revealed lower p-tau and higher Aβ42 peptide levels in the gantenerumab group.
Concerns were raised with ARIA-E, occurring in around ¼ of gantenerumab recipients, with symptomatic cases in a total of 5.0%.
In summary, while gantenerumab exhibited a reduction in amyloid plaque burden, this did not translate into a significant slowing of clinical decline in early Alzheimer’s disease over the course of 116 weeks.
This article refers to:
Bateman RJ, Smith J, Donohue MC, Delmar P, Abbas R, Salloway S, Wojtowicz J, Blennow K, Bittner T, Black SE, Klein G, Boada M, Grimmer T, Tamaoka A, Perry RJ, Turner RS, Watson D, Woodward M, Thanasopoulou A, Lane C, Baudler M, Fox NC, Cummings JL, Fontoura P, Doody RS (2023) Two phase 3 trials of gantenerumab in early Alzheimer’s disease. N Engl J Med 389:1862–1876. DOI: 10.1056/NEJMoa2304430
Further references:
[1] van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T (2023) Lecanemab in early Alzheimer’s disease. N Engl J Med 388:9–21. DOI: 10.1056/NEJMoa2212948
[2] Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, Wessels AM, Shcherbinin S, Wang H, Monkul Nery ES, Collins EC, Solomon P, Salloway S, Apostolova LG, Hansson O, Ritchie C, Brooks DA, Mintun M, Skovronsky DM (2023) Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330:512–527. DOI: 10.1001/jama.2023.13239